For CLinicans
A Groundbreaking Innovation in Drug-free Weight Management
Revolutionizing healthy weight management through science and technology, the Epitomee® Capsule is an FDA-cleared, drug-free capsule designed to facilitate weight management by promoting satiety through a mechanical, rather than pharmacological mechanism.
View IFUs

Who is the epitomee capsule For
Epitomee® is indicated to aid in weight management in overweight and obese adults with a BMI of 25 - 40 kg/m2, when used in conjunction with diet and physical activity.
See further detailed information in the Epitomee® Capsule instruction of use including contraindications and precautions sections.
The Epitomee® Capsule offers a gentle, non-pharmaceutical approach to weight management. Its unique capsule works through natural mechanism of action with your body—no stimulants, no injections, and no surgical procedures. Just simple, science-backed support for feeling full and eating less.
Response to available obesity treatments varies between individuals. Whether due to side effects or contraindications, some patients may not be suitable candidates for certain therapies. The Epitomee® Capsule provides an effective, drug-free alternative—helping you manage weight through natural mechanism of action, without compromising comfort or safety. Epitomee is a prescription device and should be taken under the supervision of a healthcare provider.
The Epitomee® Capsule may be considered as part of an overall weight management strategy, under the supervision of a healthcare provider with a non-drug solution that supports appetite control and portion reduction—so you can transition smoothly.
The Epitomee® Capsule offers a drug-free solution for patients who have difficulty managing portions. The capsule expands in the stomach to trigger a natural feeling of fullness, helping reduce intake and support weight management over time, without relying on medication.
Not every patient meets the criteria for GLP-1 medications due to BMI thresholds, contraindications, or medical history. Consult your physician about taking Epitomee, a prescription device, offers a safe, drug-free alternative designed to help these individuals manage weight through natural appetite control and portion reduction, without the need for injections or pharmaceuticals.
trusted by CLINICIANS
Backed by Experts in Weight Management

I’m especially excited about Epitomee as an option for patients who struggle with hunger control at specific times of the day-particularly in the evening, which is a common challenge. Many individuals report good appetite control during the day but experience a resurgence of cravings or loss of dietary control in the late afternoon or evening. In these cases, Epitomee may offer satiety support during these high-risk periods, helping patients better adhere to their nutritional plans.


Epitomee offers a flexible, safe, and well-tolerated weight management solution suitable for a wide range of patients across different stages of their weight loss journey. It is an outstanding addition to the army of medications and medical devices that we have today.


I see the Epitomee capsule as an important tool in my toolbox due to its promising weight management treatment. Individuals who struggle with portion control or early satiety and have had little success with diet and exercise are good candidates for Epitomee treatment.

Low Risk
Zero Related Serious Adverse Events5
The Epitomee® Capsule is designed to provide effective results with favorable safety profile. Adverse events reported by participants were mostly mild and similar between the Epitomee® and placebo groups.

Reduce risk of METABOLIC SYNDROME
Weight Loss is Associated with Reduced Risk of Metabolic Syndrome1
Weight Loss with the Epitomee® Capsule can potentially improve glycemic control and other cardiometabolic risk factors, contributing to improved overall health.
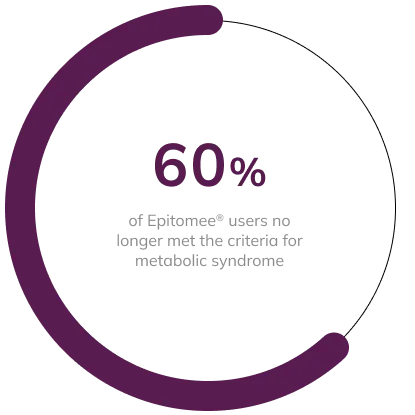
Of the 27 participants in the Epitomee® Capsule group with MetS at baseline, 16 (60%) no longer had 3 or more diagnostic components of the MetS at week 24. Among participants with MetS, those taking the Epitomee® Capsule lost significantly more weight than those on placebo (8.3% vs. 5.2%; p < 0.0004).
Improved insulin levels
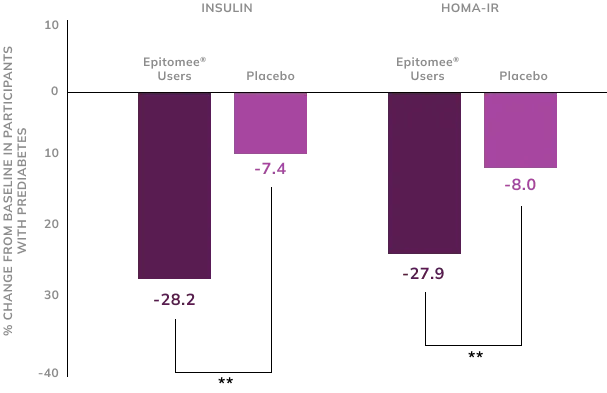
Weight Loss Helps Reduce Risk of Diabetes1
Weight loss in clinical study participants with prediabetes was associated with significant improvements in insulin levels and insulin resistance (HOMA-IR) within 24 weeks, reducing their risk of developing diabetes compared to those receiving a placebo.
**p<0.01
Predictable Weight Loss
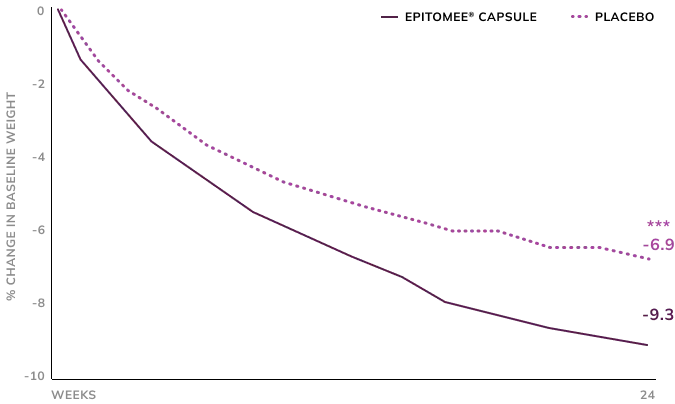
Early Responders Lost 9.3% of Their Body Weight Over 24 Weeks2
Early weight loss with Epitomee® treatment (losing at least 2% at week 8) resulted in an average weight loss of 9.3% within 24 weeks.
*** p<0.001 Epitomee versus placebo early responders
Predictable Weight Loss
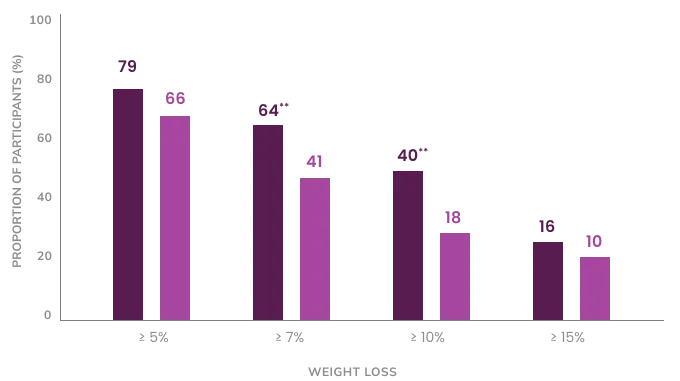
Participants Achieved a Clinically Significant Weight Loss of 5%2
79% of participants who early response to Epitomee® treatment (losing at least 2% at week 8) achieved a clinically significant weight loss of 5% within 24 weeks.
** p<0.01 Epitomee versus placebo early responders
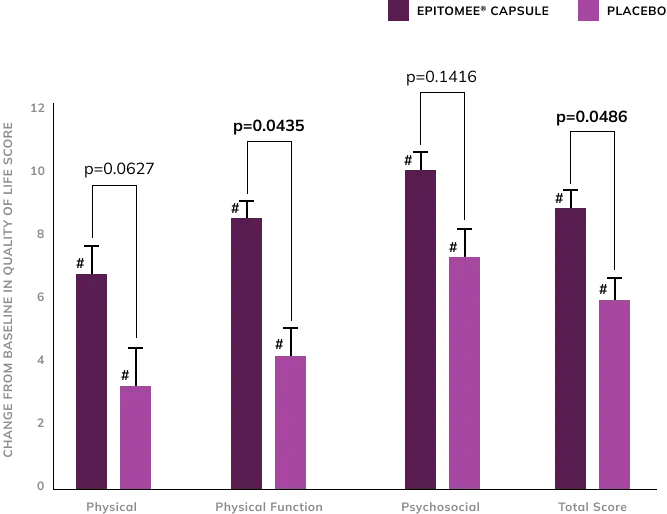
IMPROVED Quality of life
Experience Measurable Improvement In Quality of Life6
Weight loss in both Epitomee® participants and placebo significantly improved their quality of life from baseline (# p < 0.05). However, compared to placebo, the Epitomee® Capsule group exhibited significantly greater improvements in Physical Function and IWQOL-Lite-CT total scores (*p < 0.05).
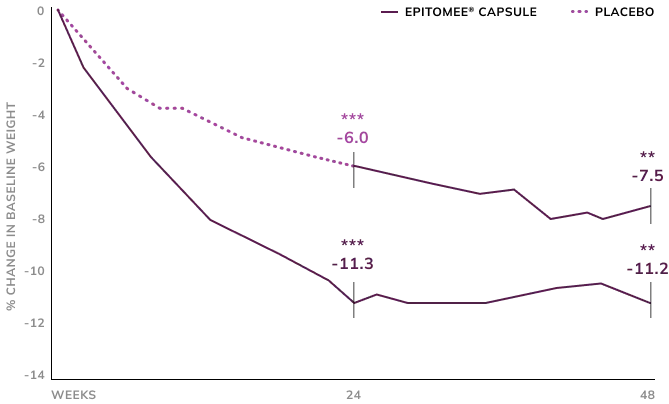
Sustainable Weight Loss
48 Week Treatment was Associated with Sustained Reduction of 11.2% Weight Loss in Ext. Study3
Participants who completed 24 weeks of treatment with Epitomee® and lost at least 3%, joined the extension study for additional of 24 weeks and had lost 11.3% of baseline body weight after 24 weeks. With 24 additional weeks of Epitomee® and lifestyle counseling, these participants maintained a loss of 11.2% of baseline body weight at week 48.
Those on placebo lost 6.0% after 24 weeks. After switching to theEpitomee® Capsule, they lost an additional 1.5% over the next 24 weeks.
**p<0.01; ***p<0.001
low risk
Safe for Extended Use3
The Epitomee® Capsule safety profile is maintained during extended use.
- 48 weeks of treatment with Epitomee® was not associated with an increase in the overall incidence of adverse events compared to 24 weeks of treatment
- No reported cases of intolerance following 48 weeks of treatment
- No serious adverse events were reported in the extended use period

HCP Brochure
Download our HCP brochure
Learn more about the science, clinical studies, benefits, and ideal patients—download our HCP brochure.
Instructions for use
Download our Instructions for use
Download our IFU to learn more about usage guidance in your selected region.

- Bays HE, Ard JD, O’Neil PM, et al. Weight and cardiometabolic effects of a novel oral shape-shifting superabsorbent hydrogel capsule: Prespecified and exploratory analysis of the Epitomee capsule RESET Study. Obes Pillars. 2025.
- Jakicic JM, Ryan DH, Ard JD, et al. Association of the Early Response to an Oral Shape-Shifting Superabsorbent Hydrogel Capsule with Weight Loss. Clin Obes. 2025.
- Kamar M, Ryan DH, Leonard S, et. al. The safety and efficacy of extended use of an oral shape-shifting superabsorbent hydrogel capsule for weight loss: the ELECT extension study. Obes Pillars. 2025; In Review.
- Shirin H, Neeland IJ, Ryan DH et al. Effects of an oral biodegradable device used for 12 weeks on weight reduction, cardiovascular risk factors, satiety, snacking, and meal size. Obesity Pillars. 2023.
- Ard, JD, Ryan DH, O’Neil PM, et al. Efficacy and safety of a novel oral hydrogel capsule in adults with overweight or obesity: the pivotal randomized RESET study. Obesity, 2025
- Kushner RF, Ard JD, Wadden TA, et al. Quality of Life Improvements Associated with Weight Loss Using a Novel Shape-Shifting Hydrogel Capsule: RESET Study Results. International Journal of Obesity. 2025.




