Clinical Results
Proven Science, Proven Clinical Results
For individuals who have struggled with portion control, GLP-1 intolerance, or weight rebound, the Epitomee® Capsule offers a science-backed solution to help reduce caloric intake while supporting lifestyle changes.
Predictable Weight Loss
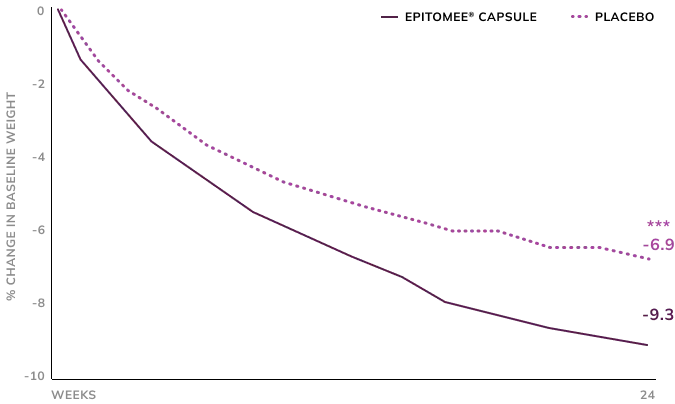
Early Responders Lost 9.3% of Their Body Weight Over 24 Weeks2
Early weight loss with Epitomee® treatment (losing at least 2% at week 8) resulted in an average weight loss of 9.3% within 24 weeks.
*** p<0.001 Epitomee versus placebo early responders
Sustainable Weight Loss
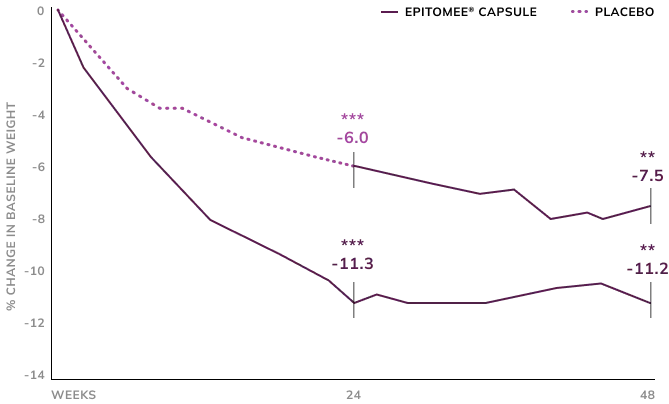
48 Week Treatment was Associated with Sustained Reduction of 11.2% Weight Loss in Ext. Study3
Participants who completed 24 weeks of treatment with Epitomee® and lost at least 3%, joined the extension study for additional of 24 weeks and had lost 11.3% of baseline body weight after 24 weeks. With 24 additional weeks of Epitomee® and lifestyle counseling, these participants maintained a loss of 11.2% of baseline body weight at week 48.
Those on placebo lost 6.0% after 24 weeks. After switching to theEpitomee® Capsule, they lost an additional 1.5% over the next 24 weeks.
**p<0.01; ***p<0.001
Reduced Waistline
Reduce Waistline by 2 Jeans Sizes over 24 Weeks1
Participants treated with the Epitomee® Capsule reduced their waistline by 2-3 inches (6 cm), equivalent to 2 jeans sizes in the first 24 weeks.

Boost satiety
Easier to Control Appetite Over 12 Weeks4
Participants steadily lost weight week by week, and showed they felt:
- Fuller sooner
- Less need to snack between meals
- More satisfied with smaller portions

Low Risk
Zero Related Serious Adverse Events5
The Epitomee® Capsule is designed to provide effective results with favorable safety profile. Adverse events reported by participants were mostly mild and similar between the Epitomee® and placebo groups.

low risk
Safe for Extended Use3
The Epitomee® Capsule safety profile is maintained during extended use.
- 48 weeks of treatment with Epitomee® was not associated with an increase in the overall incidence of adverse events compared to 24 weeks of treatment
- No reported cases of intolerance following 48 weeks of treatment
- No serious adverse events were reported in the extended use period

Reduce risk of METABOLIC SYNDROME
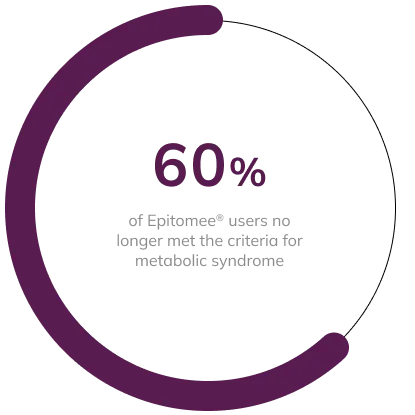
Weight Loss is Associated with Reduced Risk of Metabolic Syndrome1
Weight Loss with the Epitomee® Capsule can potentially improve glycemic control and other cardiometabolic risk factors, contributing to improved overall health.
Of the 27 participants in the Epitomee® Capsule group with MetS at baseline, 16 (60%) no longer had 3 or more diagnostic components of the MetS at week 24. Among participants with MetS, those taking the Epitomee® Capsule lost significantly more weight than those on placebo (8.3% vs. 5.2%; p < 0.0004).
Improved insulin levels
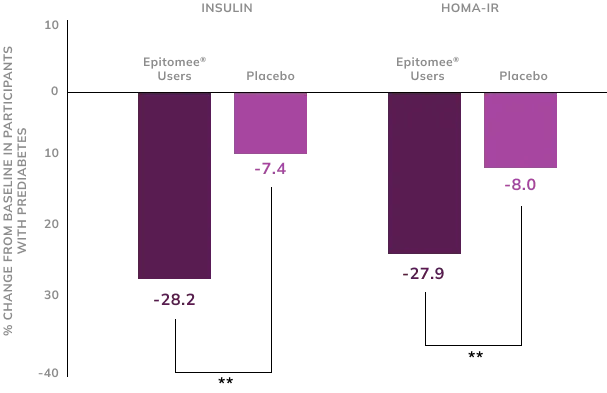
Weight Loss Helps Reduce Risk of Diabetes1
Weight loss in clinical study participants with prediabetes was associated with significant improvements in insulin levels and insulin resistance (HOMA-IR) within 24 weeks, reducing their risk of developing diabetes compared to those receiving a placebo.
**p<0.01
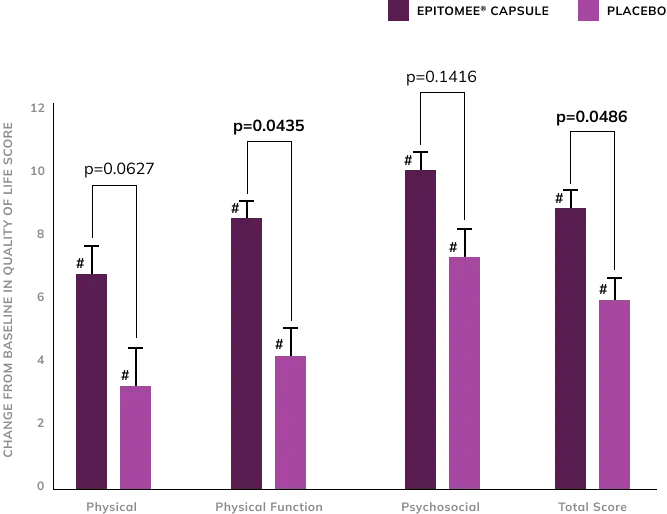
IMPROVED Quality of life
Experience Measurable Improvement In Quality of Life6
Weight loss in both Epitomee® participants and placebo significantly improved their quality of life from baseline (# p < 0.05). However, compared to placebo, the Epitomee® Capsule group exhibited significantly greater improvements in Physical Function and IWQOL-Lite-CT total scores (*p < 0.05).
- Bays HE, Ard JD, O’Neil PM, et al. Weight and cardiometabolic effects of a novel oral shape-shifting superabsorbent hydrogel capsule: Prespecified and exploratory analysis of the Epitomee capsule RESET Study. Obes Pillars. 2025.
- Jakicic JM, Ryan DH, Ard JD, et al. Association of the Early Response to an Oral Shape-Shifting Superabsorbent Hydrogel Capsule with Weight Loss. Clin Obes. 2025.
- Kamar M, Ryan DH, Leonard S, et. al. The safety and efficacy of extended use of an oral shape-shifting superabsorbent hydrogel capsule for weight loss: the ELECT extension study. Obes Pillars. 2025; In Review.
- Shirin H, Neeland IJ, Ryan DH et al. Effects of an oral biodegradable device used for 12 weeks on weight reduction, cardiovascular risk factors, satiety, snacking, and meal size. Obesity Pillars. 2023.
- Ard, JD, Ryan DH, O’Neil PM, et al. Efficacy and safety of a novel oral hydrogel capsule in adults with overweight or obesity: the pivotal randomized RESET study. Obesity, 2025
- Kushner RF, Ard JD, Wadden TA, et al. Quality of Life Improvements Associated with Weight Loss Using a Novel Shape-Shifting Hydrogel Capsule: RESET Study Results. International Journal of Obesity. 2025.




